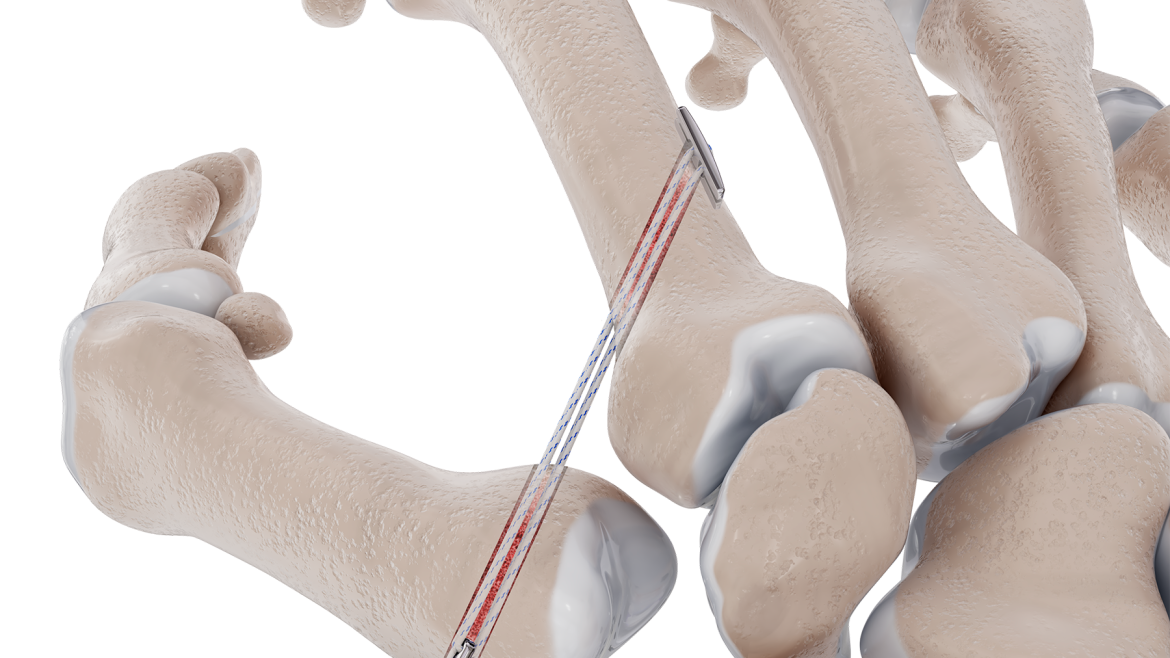
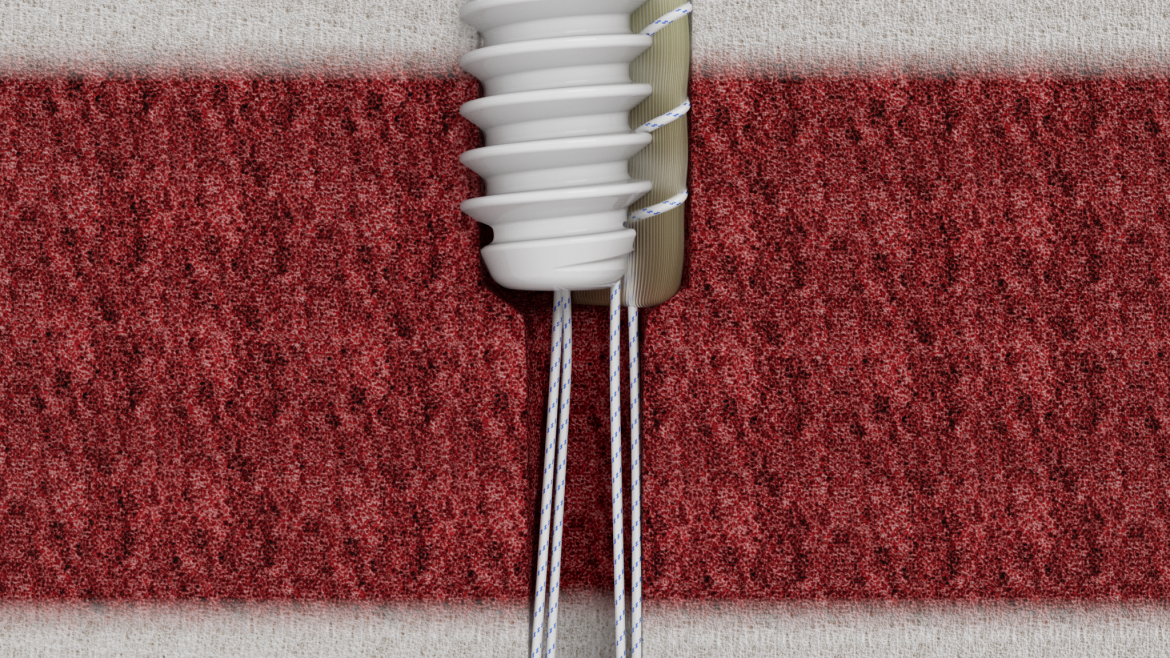
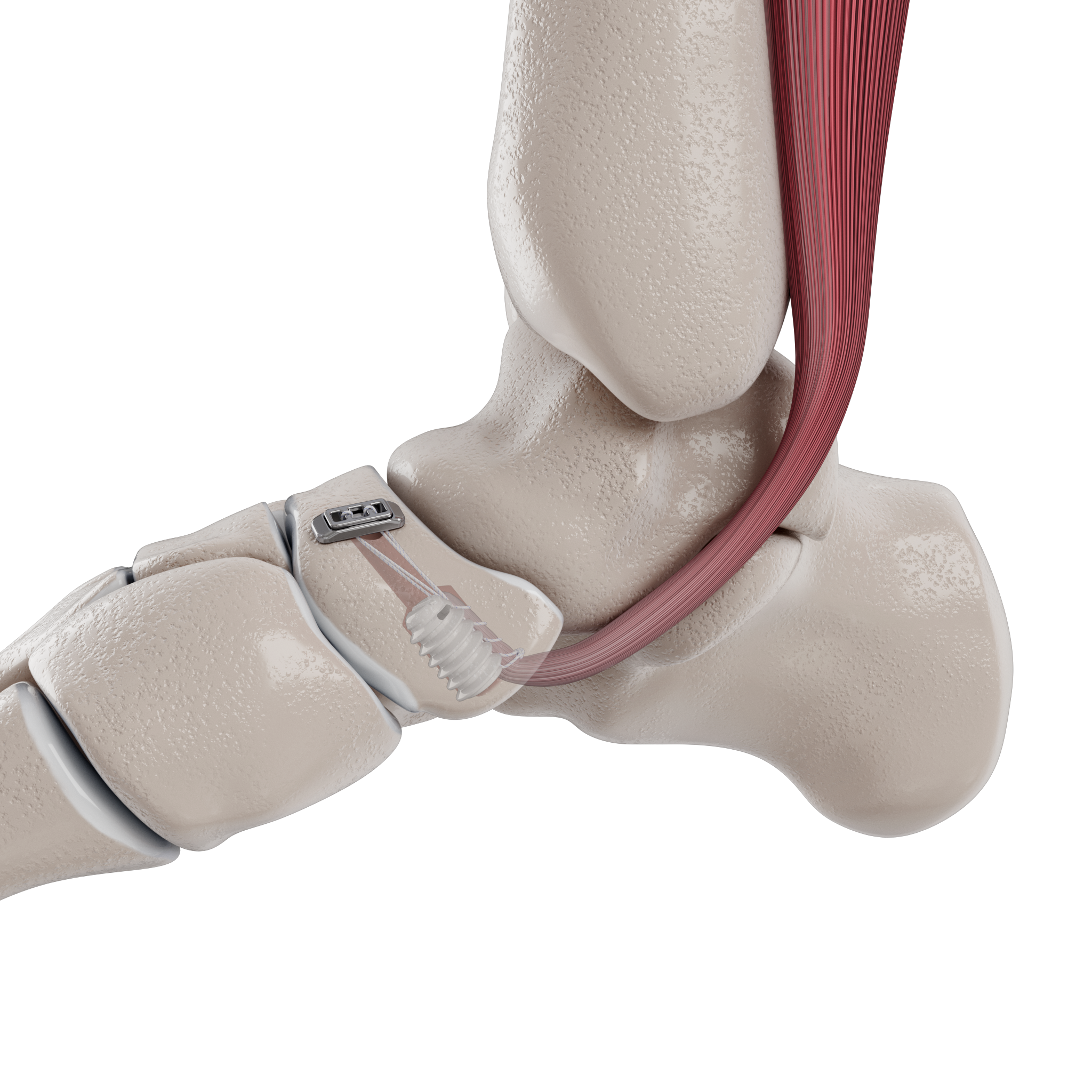
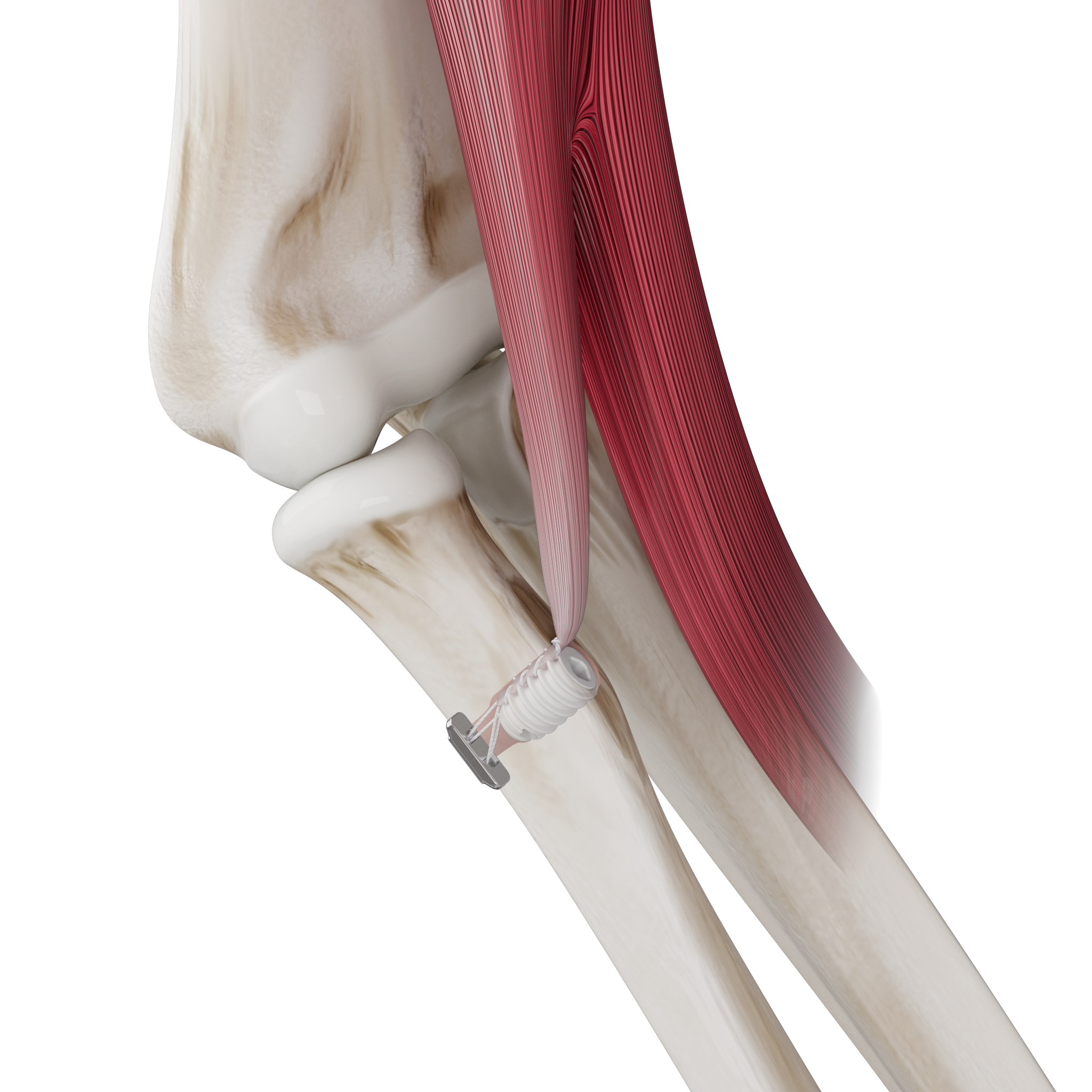
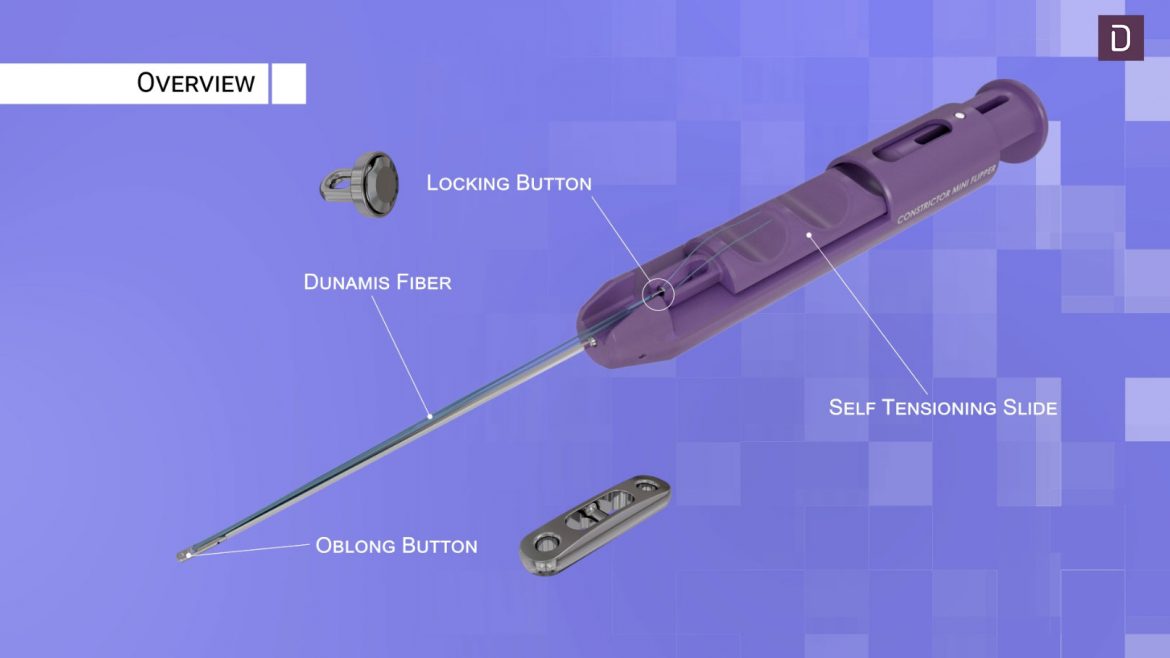
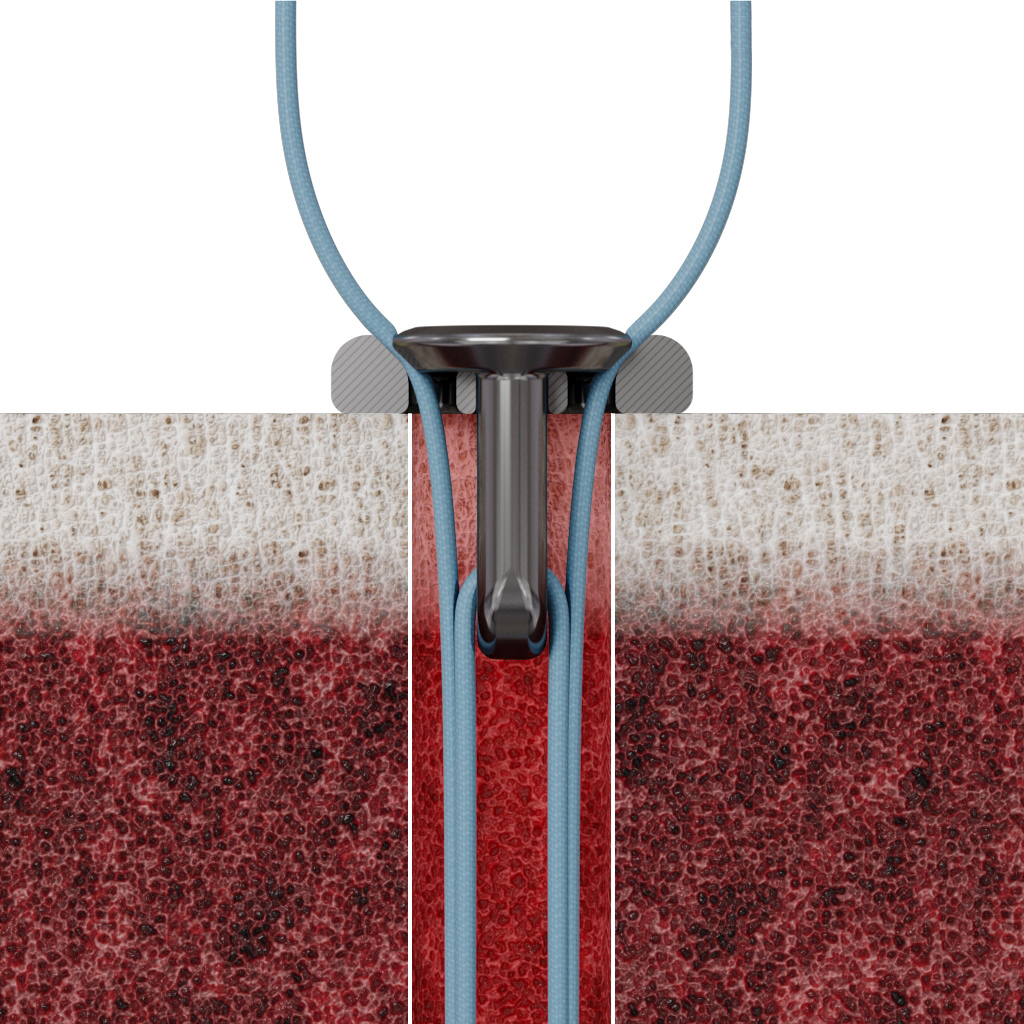
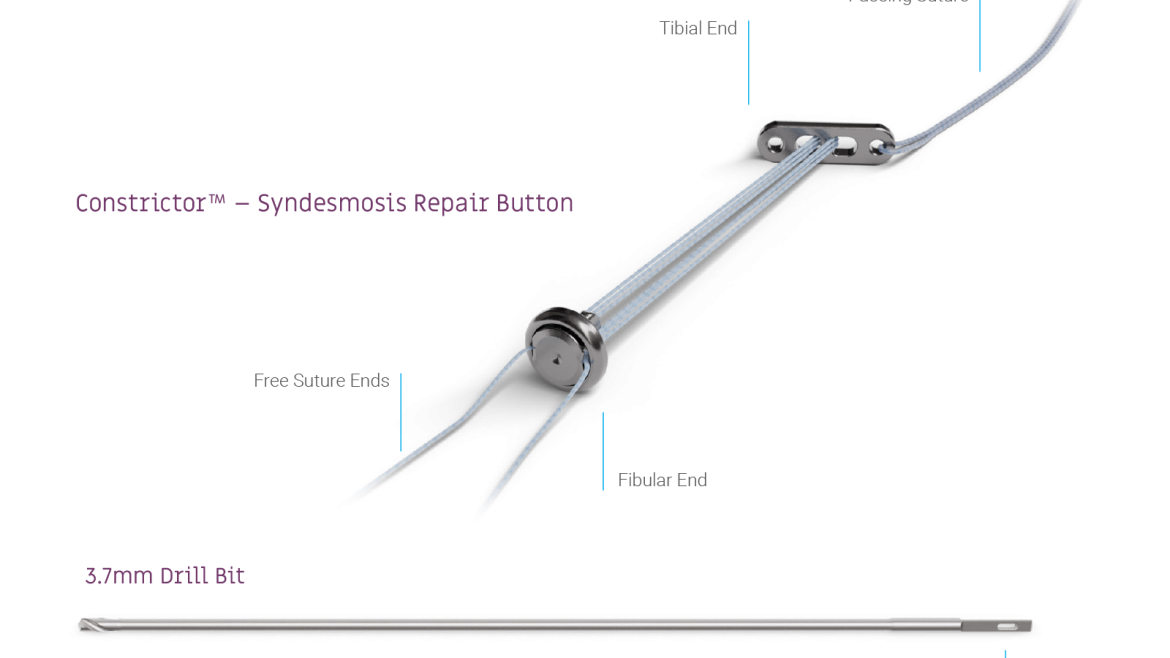
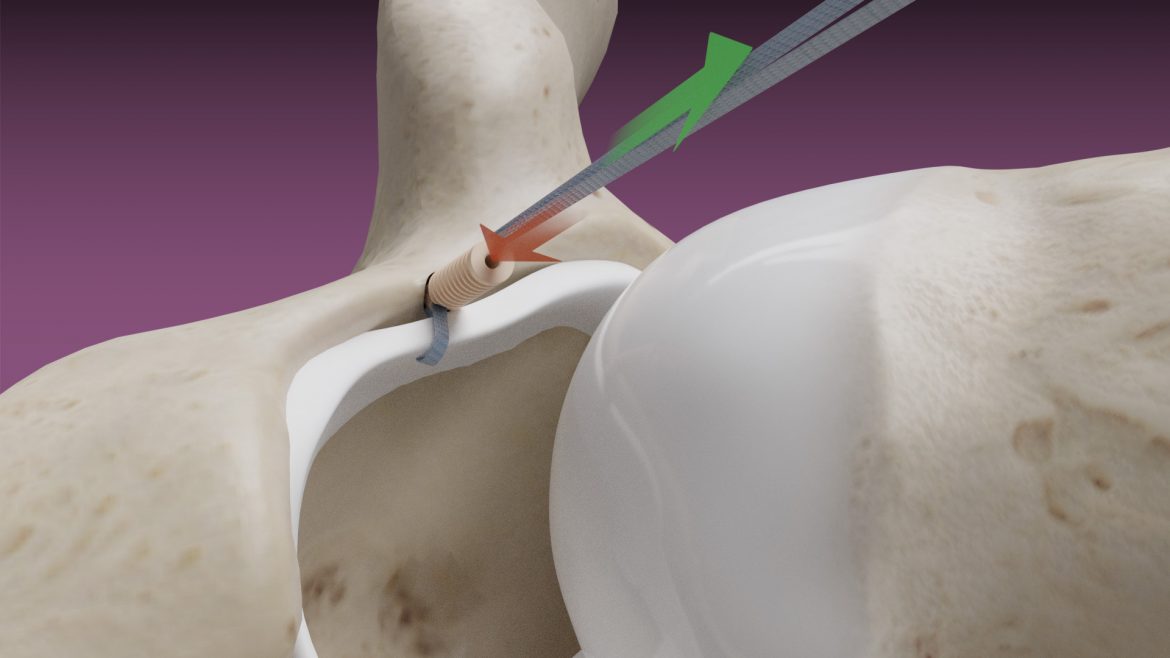
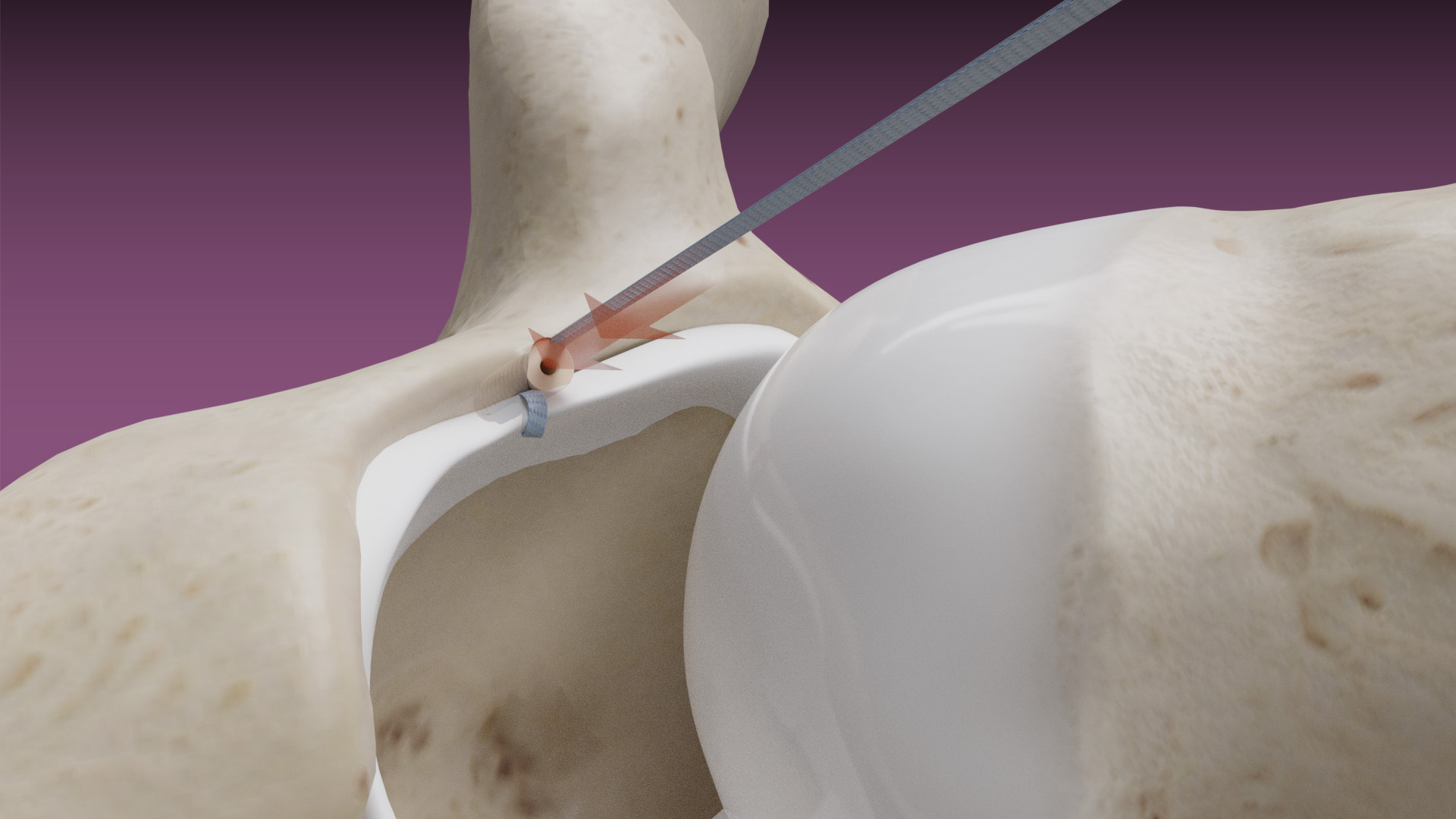
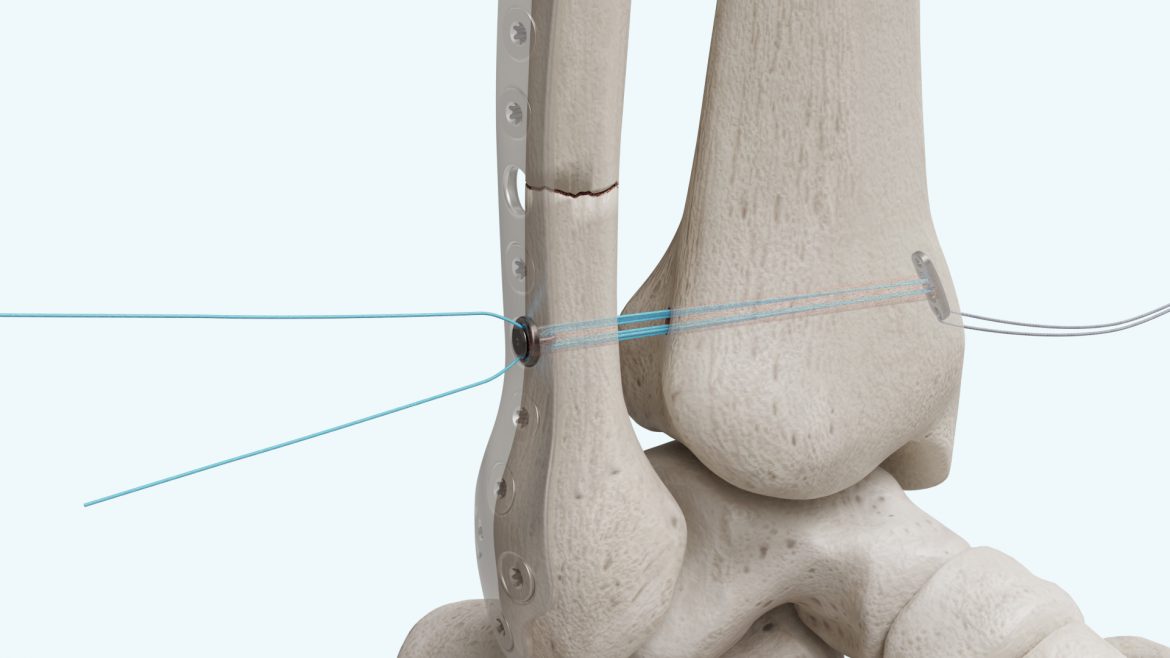
The Micro Constrictor® Knotless adjustable button offers surgeons a low-profile solution for small bone fixation, presenting an appealing alternative to traditional fixation techniques that require knot tying. This system streamlines the surgical process with a guide and spacer system, enhancing stability during the drilling and tensioning process. The Micro Constrictor® for CMC Arthroplasty provides surgeons with a robust fixation designed to promote quicker recovery and prevent proximal migration of the thumb.
“Thumb CMC OA impacts 8% of men and 25% of women older than 50. 20% of them seek some form of treatment for pain relief. With Micro Constrictor®️, our objective was to design the smallest self-locking solution for treatment of CMC arthritis. The system lets the surgeon precisely tension the repair without overtightening between the metacarpal bones. This technology creates an opportunity for our expansion into the hand market with focus on small bone fixation.” – Prithviraj Chavan, MD, Chief Executive Officer
Dunamis Medical® is committed to providing healthcare professionals with the most advanced and effective medical devices to improve patient outcomes. The introduction of the Micro Constrictor® is a testament to this commitment, and we are confident that it will become the preferred choice for orthopedic surgeons.
The Micro Constrictor® is now available for purchase. For more information, please visit https://dunamismedical.com/products/micro-constrictor/ or contact info@dunamismedical.com.”