For Immediate Release
Contact: info@dunamismedical.com
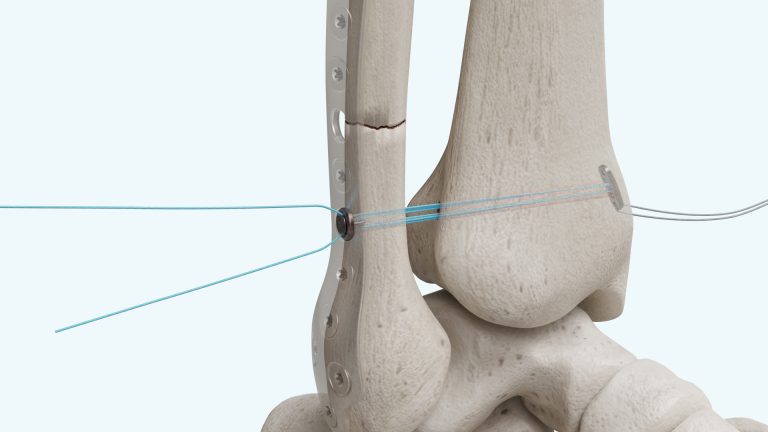
Greenville, AL – August 29, 2019 – Dunamis Medical announces FDA clearance of its Adjustable Fixation Technology for Soft Tissue Repair-Reconstruction. The Adjustable Fixation Technology is knotless, adjustable, and has a self-locking design to assist with graft tensioning. It has superior performance on cyclic loading as compared to the market leader.
Surgeons have struggled with inadequate graft tensioning which causes graft laxity, recurrent diastasis as well as loss of the repair. Devices that require knot tying are also associated with knot related complications.
Our Knotless Adjustable Fixation Technology solution uses a novel self-locking design to assist with graft tensioning after implantation. The unique locking feature can prevent graft laxity and loss of reduction. The knotless component also prevents knot tying related complications.
“The approval of our Knotless Adjustable fixation platform technology is a result of more than 3 years of commitment to product innovation and development. Our solution provides a technological advancement and marks our entry into the flexible fixation market,” said President and CEO Prithviraj Chavan, MD.
Dunamis Medical plans to begin an alpha launch by collaborating with surgeons in multiple medical centers. Our primary focus will be on Syndesmosis repair, AC repair, and ACL reconstruction.
For additional information on approved indications, contraindications, warnings, and potential adverse effects contact info@dunamismedical.com.